Ice is your friend.Oxygenators for live Wells are not needed. Why? It is physically impossible to add more oxygen to water at a given temperature. Colder water holds more oxygen than warmer water. The excess oxygen escapes into atmosphere. The oxygenator would be useful if you were not adding fresh water from the surrounding water. You want to get more O2 in the water, esp in summer, cool the water in live well w/ ice so it can hold more oxygen.
What Affects Oxygen Solubility?
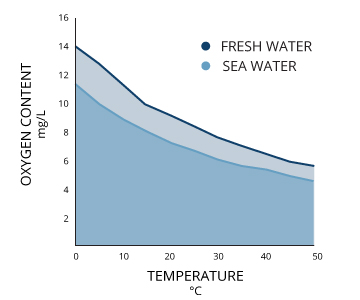
Two bodies of water that are both 100% air-saturated do not necessarily have the same concentration of dissolved oxygen. The actual amount of dissolved oxygen (in mg/L) will vary depending on temperature, pressure and salinity ¹.Dissolved oxygen concentrations decrease as temperature increases
First, the solubility of oxygen decreases as temperature increases ¹. This means that warmer surface water requires less dissolved oxygen to reach 100% air saturation than does deeper, cooler water. For example, at sea level (1 atm or 760 mmHg) and 4°C (39°F), 100% air-saturated water would hold 10.92 mg/L of dissolved oxygen. ³ But if the temperature were raised to room temperature, 21°C (70°F), there would only be 8.68 mg/L DO at 100% air saturation ³.
Source:Fundamentals of Environmental Measurements




 Reply With Quote
Reply With Quote

